Let’s be real: we all thought the future would bring flying cars, robot butlers, and maybe a casual Mars vacation. But instead, science hit us with something way more intense—a real-life gene-edited baby. Yeah, like… someone actually edited a human embryo’s DNA, then boom: baby. Born. Breathing. Living proof that science fiction just slid into our DMs and made itself at home.
The first-of-its-kind CRISPR baby was born in 2018 in China, and ever since, the medical world’s been spiraling—in awe, in panic, in deep philosophical debate. Because once you’ve cracked open the code of life itself, there’s no stuffing the DNA genie back in the bottle. So what does this actually mean for the future of medicine (besides the possibility of making super babies with flawless skin and no lactose intolerance)? Here are 12 wild, hopeful, and slightly terrifying ways gene editing could change everything.
1. Curing Genetic Disorders

Imagine a world where Huntington’s, cystic fibrosis, or sickle cell anemia are names your grandparents mention, not diagnoses you fear. Gene editing theoretically zaps out faulty DNA before it ever causes trouble. By rewriting the genetic script in embryos, we could essentially pre-program babies to be free of certain inherited conditions. According to The Guardian, the technology behind CRISPR-Cas9 has already shown promise in lab-dish models of muscular dystrophy and blood disorders—and that was years ago! It’s like having a molecular eraser that targets typos in your body’s instruction manual. Critically, though, we’re not talking DIY biohacking in your garage; clinical protocols, safety checks, and ethical reviews are mandatory pit stops on this road trip.
Of course, editing embryos raises questions about off-target effects, mosaicism, and the long-term health of those babies. Researchers are racing to refine CRISPR so that edits hit only the intended spot—think of it as upgrading from a chainsaw to a surgical laser. And babies born from edited embryos would need lifetime monitoring, because genomic tweaks can echo in unexpected ways. But if we nail the safety profile, families who’ve carried recessive genetic burdens for generations could finally exhale. Plus, insurance companies might (hopefully) start covering preventive genomic therapies, shifting healthcare spending from crisis management to true prevention. It’s not just about adding years to life, but life to years—imagine celebrating milestones without the shadow of a hereditary illness.
2. Hyper-Personalized Medicine

Move over, one-size-fits-all prescriptions—gene editing paves the way for healthcare tailored to your exact DNA footprint. Per Technology Review, researchers are already mapping how individual genetic variants affect drug metabolism, so future doctors could tweak genes to optimize your response to medications. No more trial-and-error with blood thinners or antidepressants—your genome would come with built-in dosage instructions.
Beyond pills, this approach could inform personalized cancer vaccines, immune therapies, and even nutrition plans based on how your body processes fats and carbs. Imagine opening your health app and seeing “Hey, your edited genome says broccoli is basically superfood gold for you—enjoy that extra serving!” Sure, the data privacy debates will rage on—who owns your genomic data, you or the companies analyzing it?—but in theory, we’ll sidestep adverse drug reactions and dramatically improve treatment efficacy. Think of it as the ultimate tailored suit: all the benefits, none of the awkward fit.
3. Organ Transplants on Demand
Thanks to CRISPR’s knack for precise cuts and edits, scientists could engineer pigs or other animals to grow human-compatible organs. As noted in Nature, teams have already knocked out retroviral sequences in pig genomes to reduce transplant rejection risks. The first gene-edited baby proves we can handle edits safely in humans, which bolsters confidence in more complex interspecies edits.
In a few years, dialysis lines and liver failure waiting lists might seem archaic. Instead, a patient’s cells could be used to program an organ factory—no more frantic organ matching or black-market brokers. The process would start with editing the animal’s DNA to express human proteins and remove antigens that trigger immune attacks. Then you’d simply schedule your transplant like any other elective procedure—pre-op jitters, a TikTok-ready recovery montage, and a brand-new organ ready to go. Sure, animal-rights advocates and ethicists will have opinions (and protests), but for those teetering on death’s doorstep, this looks like a game changer.
4. Tackling Infectious Diseases at the Genetic Level

What if we could engineer babies with built-in immunity to HIV, malaria, or even future viral nasties? The Harvard School Of Public Health even commented on how gene editing might one day confer resistance to pathogens that have plagued humanity for centuries. By editing genes like CCR5—which HIV uses to infiltrate immune cells—we could create an innate firewall against infection.
This isn’t just theoretical: in adults, experimental CRISPR therapies have shown promise in knocking out CCR5 in T cells to control HIV load. Extending that to embryos means you’d get a head start on immunity before your first cold. Think of it as a genetic vaccine, wired in at conception. Of course, we must guard against unintended consequences—CCR5 also has roles in fighting other infections, and editing one gene could cascade through immune pathways. But if done right, whole generations could be born with built-in defenses, drastically cutting transmission rates and reducing the need for lifelong antiviral regimens.
5. Advancing Cancer Prevention Strategies

Editing out cancer-susceptibility genes? It’s not as wild as it sounds. Science Direct found emerging research into editing BRCA1/2 variants to reduce breast and ovarian cancer risk—essentially swapping out a high-risk allele for a benign version. That means future generations might not need prophylactic mastectomies or intensive surveillance protocols.
This approach could extend to Lynch syndrome (colon cancer), familial adenomatous polyposis, and other hereditary cancer syndromes. By tweaking the germline, we shift from tertiary prevention (treating cancer) to primary prevention (never developing it). Critics worry about “designer babies” and where to draw lines—do you edit intelligence or athletic potential next?—but for now, the focus remains on removing ticking time bombs from our DNA. In practice, this could translate into healthcare systems reallocating billions in screening and treatment budgets toward ensuring healthy births, rather than paying for chemo suites and radiation facilities.
6. Reducing Healthcare Costs in the Long Run

Currently, treating chronic genetic diseases piles up expenses for patients, insurers, and national health systems. Imagine decades of dialysis, enzyme replacement therapies, or repeated surgeries—all replaced by a one-time edit at conception. In theory, that slash in lifetime healthcare spending frees up resources for other priorities, like mental health services or pandemic preparedness.
Economists would have to model the upfront costs of genomic screening and embryo editing, but early analyses suggest long-term savings. Fewer hospital stays, less pharmaceutical dependency, and a healthier workforce could boost productivity and tax revenues. Private insurers might lobby for coverage of germline editing, seeing it as a hedge against enormous payout liabilities. Meanwhile, public health budgets could pivot toward preventive programs, ushering in a truly proactive era of medicine.
7. Ethical and Social Implications Shake-Ups

Introducing heritable genome edits forces societies to wrestle with questions old and new: What counts as therapy versus enhancement? Who gets access, and who decides? Could we widen the gap between the genomic “haves” and “have-nots”? We’re talking about rewriting humanity’s code—no small potatoes.
Public discourse would need to broaden beyond academic journals to involve diverse communities, faith leaders, and global policymakers. International guidelines like those from UNESCO or the International Commission on the Clinical Use of Human Germline Genome Editing would gain superstar status. And grassroots movements could lobby for or against various applications, ensuring that this tech doesn’t become another tool of inequity. Ultimately, the world’s first gene-edited baby isn’t just a medical milestone—it’s a social experiment writ large.
8. Accelerating Research into Rare Diseases

For conditions affecting just a handful of families worldwide, traditional drug development often isn’t economically viable. Gene editing could offer a shortcut: once you understand the mutation, you design a CRISPR edit and test it in cellular or animal models. Translating that to human embryos could eventually prevent rare, devastating disorders before birth.
Patient advocacy groups might partner directly with research labs, crowdfunding targeted editing programs for specific diseases. Imagine a rare neuromuscular condition that affects ten kids globally—scientists could develop a bespoke edit, get fast-track regulatory review, and offer the therapy to those families. This democratizes rare disease research, transforming orphan diseases into tractable targets rather than pharmaceutical afterthoughts.
9. Boosting Our Understanding of Human Development
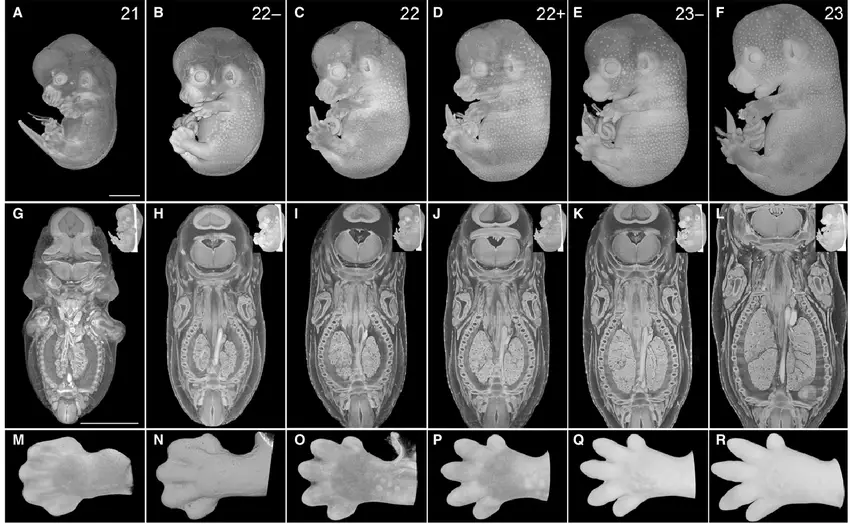
When scientists edit specific genes in embryos, they learn what each gene actually does in early development. This peek behind the curtain could illuminate mysteries like early miscarriages, congenital heart defects, or neural tube anomalies. Knowledge here doesn’t just inform editing—it enriches all of developmental biology.
Labs might generate cell lines or organoids from edited embryos to model disease, test drugs, or even explore basic questions like how brains wire themselves. That foundational insight benefits every corner of medicine, from obstetrics to neurology. It’s like turning on a floodlight in a dark room—you suddenly see all the obstacles in the way of healthy development.
10. Shaping Future Regulatory Frameworks

Countries will need to decide where to draw the line: a full moratorium, strict limitations, or an open door? The world’s first gene-edited baby will be the case study that shapes legislation everywhere. We’ll likely see a patchwork of policies—some nations embracing germline editing, others banning it outright.
Regulatory bodies like the FDA, EMA, and their counterparts in Asia and Africa will debate safety thresholds, consent protocols, and long-term monitoring. We might even see an international “Gene Editing Treaty” akin to the Non-Proliferation Treaty for nuclear weapons. This evolving legal landscape could either accelerate or stall research, depending on how nimble and consensus-driven policymakers are.
11. Inspiring the Next Generation of Scientists

Nothing grabs headlines like “First Gene-Edited Baby”—and that kind of publicity can ignite passion in aspiring researchers. High schoolers who once found biology class meh might suddenly see CRISPR as their ticket to changing the world.
We could witness a surge in genomic science programs, biotech startups, and public-private partnerships. Universities might launch specialized majors in “Genomic Ethics” or “CRISPR Engineering,” while coding bootcamps teach bioinformatics pipelines. The result? A robust pipeline of talent ready to tackle everything from climate-resilient crops to space-faring life support systems. The ripple effect on STEM fields could be enormous.
12. Redefining What It Means to Be Human

At the heart of it all, editing the germline forces us to ask: who do we want to be? Is it hubris or hope to tinker with our DNA? Are we designers, or caretakers, of our own evolution? The first gene-edited baby is more than a medical milestone—it’s a mirror reflecting our collective aspirations and anxieties.
Soon, every family history might come with a “genomic decision point,” a choice about whether to embrace editing technology. That choice will shape not just individual lives, but the human story itself. And while some may fear a slippery slope toward designer traits, others will celebrate the chance to eradicate suffering at its source. Either way, this breakthrough treatment will redefine the very essence of human potential.

